Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Covid-19 News and Discussions
- Thread starter Yommie
- Start date
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #709
Novavax Q1 2024 Earnings Preview: Success In New Commercial COVID Markets Unlikely
May 07, 2024 1:01 PM ETNovavax, Inc. (NVAX) Stock
Edmund Ingham
Investing Group Leader
Follow
Summary
- Novavax will announce its Q1 2024 earnings this Friday, 10th May.
- The company reported revenues of $476m, $1.15bn, $1.98bn, and $984m in the four years since 2020, with heavy net losses in each of those years.
- Novavax plans to focus on commercial markets and move away from advance purchase agreements, with a goal of achieving full approval for its vaccine.
- It's hard to escape the conclusion that having been second best during the pandemic era, Novavax may run into the same problems in a commercial COVID vaccine market.
- The company will update on its BLA application, preparation for the fall vaccine season, APA revenues, and its cost management program, but it's hard to see how Q1 earnings will wow Wall Street or generate upside.

J Studios/DigitalVision via Getty Images
Investment Overview
Novavax (NASDAQ:NVAX), the Gaithersburg, Maryland headquartered specialist vaccine developer will announce its Q1 2024 earnings this Friday, 10th May.Novavax is arguably best known for being the company that - very nearly - challenged the supremacy of the messenger-RNA vaccines developed by Moderna (MRNA) and Pfizer (PFE) / BioNTech (BNTX), that earned ~$135bn of revenues during the COVID pandemic era of 2021 - 2023.
Novavax received ~$1.8bn of funding from Operation Warp Speed ("OWS"), the Trump administration's vaccine accelerator program, in 2020, but despite delivering some strong safety and efficacy readouts, comparable to Moderna's SpikeVax and Pfizer / BioNTech's Comirnaty, delays to completing Phase 3 studies meant that Novavax' Nuvaxovid did not receive Emergency Use Authorisation in the US until July 2022.
By that time, both Spikevax and Comirnaty, which received their EUAs in December 2020, dominated the COVID vaccine market, and although Novavax secured approvals in the UK, Canada, Australia, Switzerland, Singapore and New Zealand, and thanks to a partnership with the Serum Institute in India, South Korea, Thailand and Bangladesh, the company could not match its rivals' earnings power, reporting revenues of $476m, $1.15bn, $1.98bn, and $984m in the four years since 2020.
Nor could Novavax return a profit in any of those years, reporting net losses of $(418m), $(1.74bn), $(658m), and $(545m) in the four years since 2020.
Prior to the pandemic, Novavax - without a product approval in the company's history - was developing vaccines against influenza and RSV, and its shares traded ~$5. During the pandemic, shares reached highs of >$170 in August 2020, >$290 in February 2021, >$250 in August 2021, and >$75 in July 2022, when the US EUA was received.
Today, however, Novavax stock is once again trading <$5, although around the time company announced its Q4 2023 and full year 2023 earnings in late February, it briefly traded >$6 per share, and after sinking <$4 per share last month, has enjoyed a small rally to current price of $4.75 per share.
The days of wild share price fluctuations and spikes >$100 per share are a long way behind Novavax now - a spike into the double digits would frankly be surprising in the current climate, as management wrestles with financial problems, announcing plans to let go ~30% of staff when announcing its 2023 earnings.
With that said, Novavax does have an effective, approvable vaccine product that may be capable of driving "blockbuster" revenues for many years to come, if a long-term private COVID vaccination market emerges, as many believe it will. As such, Novavax retains the ability to surprise the market, as nobody quite knows what to expect from the company, or how its target market may develop over the coming years.
In the remainder of this post I'll discuss what highlights are likely to be under scrutiny when Novavax announced its Q1 2024 revenues. Let's begin by looking at 2023 highlights.
Novavax 2023 Earnings Review
When I covered Novavax back in October 2023, management was guiding for full-year 2023 revenues of $1.3bn - $1.5bn, and product sales of $960m - $1.14bn. Not for the first time, the company ultimately fell well short of that guidance.Product sales in 2023 came to $531m, with $251m earned in Q4, implying some beneficial tailwinds from the fall vaccine season, but ultimately, $984m of revenues for the year - which included $427k worth of grant revenues (presumably dating back to the OWS funding) was well short of expectations. Total product sales for the year were $531m, versus $1.55bn in the prior year.
On the plus side, cost of sales for the year of $343k was down 62% year-on-year, R&D expenses of $738k down 40% year-on-year, and with SG&A flat year-on-year, at $469k, total expenses shrank from $2.62bn in 2022, to $1.55bn. With revenues down by ~$1bn year-on-year, net loss was $(545m), or $(5.4) per share, which, although an improvement on $(8.42) in 2022, highlights the financial problems faced by the company.
End of year cash position was reported as $569m, down from $1.34bn at the end of 2022, and the company reduced its convertible notes payable down from $491m, to $168m at the end of 2023.
In terms of forward guidance (whilst acknowledging that Novavax rarely meets its own guidance targets), management is forecasting for revenues of $800m - $1bn in 2024, with combined R&D and SG&A costs of $700 - $800m. Profitability in 2024 feels unlikely, however, given we must add cost of sales, tax, and other expenses to the R&D and SG&A costs.
Looking Ahead - What Can Novavax Achieve This Year - & In The Longer Term?
In 2023, Novavax earned $30m of its revenues in the US, $268m in Europe, and $233m across the rest of the world ("ROW"), compared to $194m in the US, $823m in Europe, and $537m ROW in 2022.The bulk of the company's revenues have historically been earned through advance purchase agreements ("APAs"), and in its 2023 earnings press release, Novavax states that it has:
If all such orders are executed then Novavax would exceed its 2024 guidance on APAs alone, so this is one area investors will be hoping management can provide an update on when Q1 earnings are announced this Friday.Potential APA deliveries for 2024 through 2026 of over $1 billion consisting primarily of deliveries to Australia, New Zealand, Canada, Israel and Europe
With that said, Novavax' long term plan is to move away from APAs and intensify its focus on commercial markets. As the company CEO, John Jacobs, who replaced long-time CEO Stanley Erck around one year ago, with the latter paying the price for failing to bring nuvaxovid to key markets fast enough, told an audience at the recent TD Cowen Healthcare conference:
Commenting on the proportion of revenues that would be derived from APAs in 2024, CEO Jacobs told the audience:We continue to get the majority of our revenue...from outside the US through APAs that will be ongoing through 2026. Over the next three years, we migrate away from APA to a fully commercialized marketplace by 2027.
It's very important that we begin to penetrate and perform well in commercial markets. This coming year in the US, as well as in Europe, where we move to tenders in countries like Italy and Spain that we're targeting private pharmacy markets in France and UK which we're targeting believe will have success in.
So the lessons learned certainly get beyond the rest of these first have a better presentation of our products. So we're targeting a pre-filled syringe in 2024 from a five-dose vial.
In order to succeed with a pivot into traditional commercial markets, Novavax would ideally want to secure a full approval for its drug, rather than having to apply for an EUA each time it releases a new nuvaxovid shot, targeting the latest strain. The target is determined by the Vaccines and Related Biological Products Advisory Committee ("VRBPAC") on an annual basis, and this year, VRBPAC will announce its decision this month, a month earlier than last year, giving vaccine makers more time to prepare their shots.it's 500 million to 600 million range for APA business outside of the United States. A large majority of that coming from Australia, New Zealand, Canada, but our European APA ended last year. And then you have commercial revenues, 300 million to 400 million range, you're at midpoint 350 between the US and select European countries.
Novavax management says it expects its Biologics Licence Application ("BLA") to be approved by the Food and Drug Agency ("FDA") this year, which will allow it to focus more resources on marketing and promotion as opposed to regulatory matters.

Novavax US commercial performance analysis (Q423 earnings presentation)
As we can see above, Novavax believes it will be more agile in 2024 and beyond, with a more convenient, pre-filled syringe product, a fully authorised product, more time to prepare, and better relations with health insurers and pharmacies making it easier for patients to purchase a COVID vaccination.
Similarly, goals for Europe, APAC, and Canada are to streamline the product offering, and focus on core markets only, as APAs are gradually replaced by commercial sales.
It is hard to criticise the company's strategy, but there are several risks and drawbacks to consider. Firstly, competition will be present in the form of mRNA vaccines, which the public are more familiar with, and, despite some negative publicity, will quite possibly seek out ahead of Nuvaxovid when considering getting a private vaccination.
Secondly, from an APA perspective, there is a risk that governments and agencies will renege on agreements, or that Novavax may struggle to fulfil orders, as happened with the GAVI international vaccine group. Novavax failed to deliver vaccines to GAVI within the agreed timeframe and ultimately has agreed to pay back to the agency $475m by 2028, with Gavi no longer requiring the vaccines.
Thirdly, no one truly knows what to expect from a private COVID vaccine market i.e. will consumers be prepared to pay for COVID shots, and will health insurers provide reimbursement for shots, as happens with the flu market, or will the demand for COVID vaccinations simply fizzle out, other than occasionally government buying for its elderly populations?
Novavax CEO Jacobs told the audience at TD Cowen's conference that "everyone assumed it would be roughly 25% to 35% of the market similar to flu", i.e. that 25-25% of Americans would seek out a COVID jab, but that market did not seem to materialise last year.
Moderna is guiding for $4bn of revenues from its "respiratory" vaccine franchise this year, with the vast majority of revenues occurring in the second half of the year - presumably owing to an expected large demand for fall COVID vaccinations.
Clearly, if such a market does not emerge, when its APAs are exhausted, Novavax may have an unsellable product on its hands, and if even if such a market does arrive, Novavax will once again be scrapping with the MRNA giants Moderna and Pfizer for market share.
There is nothing wrong with Novavax's vaccine per se, although it takes a little more time to prepare new versions targeting new strains, and its preparation is a little more complex in some ways - according to NebraskaMedicine.com:
The vaccine also comes with a soapbark tree extract adjuvant to increase the immunological effect, allowing a smaller overall dose to be used. During the pandemic period, Novavax struggled to successfully manufacture and distribute its product, damaging its reputation, and even though markets are much smaller today, there is still a risk the company could fall short again when taking on the MRNA giants in a private COVID market.The Novavax method uses moth cells to make spike proteins:
- Researchers select the desired genes that create certain SARS-CoV-2 antigens (spike protein).
- Researchers put the genes into a baculovirus, an insect virus.
- The baculovirus infects moth cells and replicates inside them.
- These moth cells create lots of spike proteins.
- Researchers extract and purify the spike proteins.
Concluding Thoughts - What Am I expecting From Q1 24 Earnings & Business Updates?
When it comes to Novavax, it is sometimes hard to avoid tagging the company as the "also-ran", or bridesmaid (i.e. "never the bride") of the pandemic period, a company that might have earned revenues in the double digit billions, but ultimately fell short in several key areas - pace of clinical studies, manufacturing and infrastructure, and arguably, a vaccine product that fell narrowly short of matching the MRNA vaccines for convenience, efficacy, and speed of rollout.As such, my expectations aren't especially high for the upcoming earnings. I would not expect the company to report much in the way of revenues as most of the APA agreements are likely to be settled later in the year, I'd assume, and the fall vaccination season is the key period for earnings commercial revenues.
Where the company may offer investors hope is with some positive developments in terms of the approval of its BLA in the US, and early development of its vaccine targeting the newest strain of COVID, so that it is ready to be launched and compete against its rival vaccines as early as possible.
Management will also (hopefully) update on progress with commercial and retail partners - one major deal could transform the company's fortunes, but will any company step up and make nuvaxovid a central part of its plans to compete in COVID markets?
Finally, I'd be hoping to hear more around the development of Novavax's dual COVID / influenza vaccine. In its Q4 earnings press release, management stated it expected to:
Once again, this is not a concept unique to Novavax - the likes of Pfizer / Moderna are also focused on creating such a vaccine.initiate a pivotal Phase 3 trial for COVID-19-Influenza Combination (CIC) vaccine candidate in the second half of 2024, with potential for accelerated approval and anticipated launch in 2026
With a market cap of just over $600m at the time of writing, some might call the company undervalued if it does succeed with its BLA and finds this years' fall vaccine season to be larger than last year's - the company is arguably better prepared this year than last.
I'd agree with that sentiment, however I expect the main focus of Novavax' earnings may be around cost savings and trimming expenses as the company's tries to ensure it can remain a going concern long-term.
The very real threat that when fall arrives, is that demand for COVID vaccines doesn't meet expectations to become influenza sized, i.e. ~$10bn per annum, or that demand for Nuvaxovid fails to materialise. In that event, Novavax will essentially have only a single approved product, for which there is no demand.
That will weigh heavily on the company valuation until management can prove otherwise, and since management cannot provide updates in this regard on Friday, I'd make Novavax stock a "hold". The story of Novavax remains what could have been, not what is coming, and I don't see that changing on Friday.
Gain access to all of the market research and financial analytics used in the preparation of this article plus exclusive content and pharma, healthcare and biotech investment recommendations and research / analytics by subscribing to my channel, Haggerston BioHealth.
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #710
Health systems and employers count economic cost of long Covid
Experts warn better data is required to drive policy as debilitating condition reduces workforce
 www.ft.com
www.ft.com
Please use the sharing tools found via the share button at the top or side of articles. Copying articles to share with others is a breach ofFT.com T&Cs and Copyright Policy. Email [email protected] to buy additional rights. Subscribers may share up to 10 or 20 articles per month using the gift article service. More information can be found here.
https://www.ft.com/content/bb09a03d-4a87-4cea-ae87-986769fd4680
Health systems and employers count economic cost of long CovidExperts warn better data is required to drive policy as debilitating condition reduces workforceTiko Bakhtadze, a nurse based in Tbilisi, Georgia, was one of those who suffered protracted long Covid symptoms © FT montage; WHO/Halldorsson/GettyHealth systems and employers count economic cost of long Covid on x (opens in a new window)Health systems and employers count economic cost of long Covid on facebook (opens in a new window)Health systems and employers count economic cost of long Covid on linkedin (opens in a new window)Savecurrent progress 98%Sarah Neville, Delphine Strauss, Michael Peel and Sam Fleming in London YESTERDAY72Print this pageUnlock the Editor’s Digest for freeRoula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.Long Covid is exerting a silent drag on work and health, say officials and economists who warn that a struggle to count the costs of the condition is leaving authorities “shooting in the dark”.The impact of long Covid — defined as symptoms that continue or develop three months after an initial infection, and last at least two months — has dealt a long-lasting blow to the productivity of health systems, with ripple effects on the wider workforce.But four years after the emergence of the pandemic, attempts to assess how large and enduring the hit will be are hampered by a dearth of data that accurately quantifies the effects of long Covid on the labour market and the finances of healthcare providers.“We have growing evidence that the burden of long Covid is still exacerbating pressure on our health systems,” said Hans Kluge, European regional director of the World Health Organization. “But countries are not monitoring and reporting data consistently. We need better reporting, surveillance and diagnostics, but also data on hospitalisations, mortality and healthcare costs.”Without this, he warned, “we will continue to shoot our policy bullets in the dark”. The WHO aims to determine the extent of long Covid among health workers involved in rehabilitating Covid patients in Armenia, Georgia, Italy, Poland and the UK.One EU estimate suggests that long Covid may have cut labour supply in the bloc by up to 0.5 per cent in 2022, the equivalent of more than 1mn full-time workers. Studies in the US and UK have reached broadly similar conclusions — suggesting the condition has driven the recent increase in workplace absence in many countries.But no one knows how many people who stopped or scaled back work because of long Covid have been forced to leave their jobs for good — and how many have been able to return, either in a reduced role or gradually resuming their previous responsibilities.Tiko Bakhtadze, a 36-year-old nurse based in Tbilisi, Georgia, who fell severely ill with the virus early in the pandemic, suffered protracted long Covid symptoms that meant for a period she “wasn’t as productive as I used to be”.Persistent memory problems, for example, meant she had to take detailed notes when she returned to work. Bakhtadze has now largely recovered, insisting she never let her patients down or compromised their safety.Tiko Bakhtadze said long Covid symptoms meant that for a period she ‘wasn’t as productive as I used to be’ © WHO/HalldorssonIt is far from clear how long economies will be affected. An estimated 36mn people across WHO’s European region, which covers 53 countries with a total population of almost 1bn, may have experienced long Covid symptoms in the first three years of the pandemic, said Kluge. He added that the condition’s prevalence was about 1.7 per cent of the EU population in 2021 and nearly 3 per cent in 2022.In the US, the Census Bureau’s Household Pulse Survey shows that 1.7 per cent of American adults were reporting “significant activity limitations” as a result of long Covid in February and early March this year.Robust data is sparse, however, making it hard to tell whether long Covid is a growing problem, or one mostly affecting people who fell ill early in the pandemic and have not recovered.A rare data release last month from the UK’s Office for National Statistics — which ran a fresh study of trends in self-reported Covid-19 symptoms over the winter — found that 2mn people, or 3.3 per cent of the population in England and Scotland, described themselves as having long Covid. Half of those had contracted it more than two years earlier. Among working-age adults, 0.5 to 1 per cent of the overall population said long Covid reduced their ability to carry out daily activities a lot, the data showed.The insurers Aviva and Legal & General both said long Covid claims through their income protection schemes were now too low as a share of the total to be able to provide figures.While UK business groups say employers are increasingly concerned about the rising cost of medical cover for their staff as NHS waiting lists push people towards private healthcare, long Covid does not loom large in conversations about workplace health.But the extent of the problem is not always visible to employers, as some people have left the workforce for good while others feel there is a stigma attached to long Covid, making them reluctant to disclose it to their boss.Scientific research suggests the impact of long Covid on employees could be significant — particularly from cognitive impairment, or so-called brain fog. Up to 28 per cent of people infected with Covid go on to suffer from long Covid and almost one in four of those experience brain fog, according to the latest international findings from the journal General Hospital Psychiatry. One in six UK manufacturers cited Covid-19 and self-isolation as a main reason for long-term employee absences in 2023, according to a survey of 152 companies by Make UK, an industry body.The burden may be at its most severe in healthcare. Hiring temporary workers to cover staff absences proved costly. In Germany, official data shows that in 2021, the number of temporary healthcare workers grew 8.7 per cent from a year earlier, driven largely by the need to replace health workers off sick or leaving the profession.In the UK, an estimated 5,000 to 10,000 NHS staff were off sick with long Covid in 2023, according to analysis by the BBC, while a separate study by researchers at the universities of Portsmouth and Southampton estimated that long Covid had led to 80,000 people leaving the workforce by March 2022.Matthew Taylor, chief executive of the NHS Confederation that represents health leaders across England, Wales and Northern Ireland, said “the evidence is that long Covid is slightly more prevalent among health workers” because of the greater likelihood they contracted the original “wild type” of the virus.But he added: “We’re still quite a long way from understanding the extent of the impact on the general population’s health and the resource issues generated by actually providing support for people with long Covid.”Deepening the uncertainty surrounding the condition, it is not always possible to disentangle the impact of long Covid from that of Covid itself and a myriad of other viruses, as well as the deterioration in mental health suffered by many health staff who worked through the pandemic.RecommendedThe Big ReadThe next pandemic is coming. Will we be ready?Carmen Scheibenbogen, a clinical immunologist who runs an outpatient clinic at Berlin’s Charité hospital, which specialises in long Covid and myalgic encephalomyelitis or chronic fatigue syndrome, said the average number of sick days taken by healthcare staff in Germany had doubled between 2020 and 2023.This was not solely the result of Covid — other respiratory infections such as flu and respiratory syncytial virus played a part — but even illnesses that appeared to have another cause might in reality be attributable to long Covid, she pointed out.With long Covid cases likely to be “under-reported”, a subset of patients diagnosed with problems such as depression or muscle pain were also likely to be suffering from the condition, Scheibenbogen said.The condition’s emergence offers important lessons for health systems, employers and policymakers, said David Cutler, professor of applied economics at Harvard University.“We need better treatment, helping primary care doctors treat long Covid better. We also need to help employers learn how to enable employees suffering from it to work in the most productive way possible.”Cutler added that deeper research was also required into the best therapies for people with long Covid. “It’s a lot of people, it’s a big deal, and it’s under the radar,” he said.
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #711

Scientists create vaccine with potential to protect against future coronaviruses
Researchers say experimental shot is step towards goal of creating vaccines before a pandemic has started
Scientists create vaccine with potential to protect against future coronaviruses
Researchers say experimental shot is step towards goal of creating vaccines before a pandemic has startedIan Sample Science editor
Mon 6 May 2024 10.52 BST
Share
Scientists have created a vaccine that has the potential to protect against a broad range of coronaviruses, including varieties that are not yet even known about.
The experimental shot, which has been tested in mice, marks a change in strategy towards “proactive vaccinology”, where vaccines are designed and readied for manufacture before a potentially pandemic virus emerges.
The vaccine is made by attaching harmless proteins from different coronaviruses to minuscule nanoparticles that are then injected to prime the body’s defences to fight the viruses should they ever invade.
Because the vaccine trains the immune system to target proteins that are shared across many different types of coronavirus, the protection it induces is extremely broad, making it effective against known and unknown viruses in the same family.
“We’ve shown that a relatively simple vaccine can still provide a scattershot response across a range of different viruses,” said Rory Hills, a graduate researcher at the University of Cambridge and first author of the report. “It takes us one step forward towards our goal of creating vaccines before a pandemic has even started.”
Tests in mice showed that the vaccine induced a broad immune response to coronaviruses, including Sars-Cov-1, the pathogen that caused the 2003 Sars outbreak, even though proteins from that virus were not added to the vaccine nanoparticles. Details of the work, a collaboration between the universities of Cambridge and Oxford and the California Institute of Technology, are published in Nature Nanotechnology.
The universal coronavirus vaccine can be made in existing facilities for microbial fermentation, Hills said, adding that the researchers were working with industrial partners on ways to scale up the process. The nanoparticles and viral proteins can be made at different times in different places and mixed together to produce the vaccine.
Medical regulators do not have procedures for proactive vaccinology and the researchers say these would have to be worked out with the relevant bodies. If the vaccine were found to be safe and effective in humans, one option would be to use it as a Covid booster with the added benefit of it protecting against other coronaviruses.
More likely is that countries would hold stocks of the vaccine, and others designed to target separate pathogens, once they have been manufactured and approved. “In the event that a coronavirus or other pathogen crosses over you could have pre-existing vaccine stocks ready and a clear plan to quickly scale up production if needed,” Hills said.
Prof Mark Howarth, a senior author of the study, said: “Scientists did a great job in quickly producing an extremely effective Covid vaccine during the last pandemic, but the world still had a massive crisis with a huge number of deaths. We need to work out how we can do even better than that in the future, and a powerful component of that is starting to build the vaccines in advance.”
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #712
Ottawa will stop providing COVID-19 rapid tests to regions
No plans to replenish inventory, set to expire in December, says Health Canada

Bobbi-Jean MacKinnon · CBC News · Posted: May 07, 2024 4:00 AM EDT | Last Updated: May 7

'Rapid test programming has always been and continues to be a provincial/territorial responsibility,' a Health Canada spokesperson said. (CBC)
Social Sharing
- X
comments
The Canadian government plans to stop supplying provinces and territories with free COVID-19 rapid tests, which has an infection control epidemiologist worried about two-tiered health care, increased spread and increased health-care costs.
"The federal government continues to support Canada's rapid testing needs while the federal inventory remains," Health Canada spokesperson Nicholas Janveau told CBC News.
"That said, rapid test programming was and continues to be a provincial/territorial responsibility."
Ottawa currently has about 70 million of the tests, which people can use at home to screen for the virus. About 3.6 million of these have already expired and are ineligible for distribution.
The tests usually come in boxes of five, which would mean an inventory of just over 13 million test kits.
Canada's estimated population, as of Jan. 1, is nearly 41 million, according to Statistics Canada.Given the current COVID-19 outlook, inventory levels, and indicated testing demands, the federal government does not anticipate the need for additional federal procurements at this time.- Nicholas Janveau, Health Canada spokesperson
While Health Canada has authorized extending the shelf life of some rapid tests, all of the tests in the federal inventory will expire by December, said Janveau.
"Given the current COVID-19 outlook, inventory levels, and indicated testing demands, the federal government does not anticipate the need for additional federal procurements at this time," he said in an emailed statement.
Public health should not be based on 'ability to pay'
Infection control epidemiologist Colin Furness, a self-described "early and strong proponent of rapid tests," who has spent years saying more resources are needed to fight the pandemic, said he's not surprised Ottawa wants to "get out of the testing game" and doesn't blame the federal government since health care is a provincial and territorial responsibility.The problem, said Furness, is that if the jurisdictions don't step up to provide free, or at least subsidized tests, people will be forced to buy them if they want to know whether they're COVID-positive and should take measures to prevent transmission. And this creates a divide.

Colin Furness, an infection control epidemiologist and associate professor at the University of Toronto, contends free rapid tests should continue to be available through provinces and territories because there are still enough people who care if they're COVID-positive and want to avoid getting anyone else sick. (Katarina Kuruc)
"Public health should not be based on your ability to pay," said Furness, an associate professor at the University of Toronto.
Some people can afford to buy rapid tests, available at some pharmacies, stores and online for about $7 plus tax per test, but "many can't."
"I think we should be very cognizant that rapid tests are part of what makes us healthy. It's part of health care. It's a diagnostic [tool] and it just doesn't make sense to commodify it," said Furness. "It's just going to create sickness and sickness is expensive for everybody."
N.B. to determine next steps for its program
At least one province is mulling the future of its COVID-19 rapid point-of-care testing program. Last week, New Brunswick said demand for the tests has declined steadily since last fall, and the province is "determining next steps."New Brunswick has an adequate supply of the tests, which are all due to expire in September, said Department of Health spokesperson Sean Hatchard.
He did not say how many, but the federal government's website shows New Brunswick had an estimated inventory of 147,000 tests from Ottawa, as of last June — the smallest stockpile in the country. The department has previously said it has an additional reserve of tests, however, beyond what is reported on the federal website.
Hatchard did not say if the province plans to order any more.
Lack of public health policies, messaging
Furness said it's no surprise demand for rapid tests has dropped because public health officials across the country aren't telling people to test."They're going along with the narrative that really there isn't any more COVID, or very little, and this is not something you need to worry about," he said.
Similarly, there are no COVID policies, said Furness.
Public health officials have been encouraging people to take responsibility for their own health but a lack of available COVID-19 tests will interfere with that, argued Furness. (CBC)
"I mean, what good is it to test positive if you still have to go to work because you need to feed your family and you don't have any paid sick days, right?
"What good is a rapid test when public health guidance says, 'Well, as long as you feel pretty good and you're not coughing too hard, you should go ahead and go to work?'"
Stay home when sick, regardless of testing
Testing is an important tool to limit the spread of COVID-19, along with personal protective measures and vaccination, the Health Canada spokesperson acknowledged."Rapid tests may be used to quickly identify if you have COVID-19, and isolate if the result is positive," said Janveau.
Still, the Public Health Agency of Canada recommends anyone who feels sick or has COVID-like symptoms stay home and limit their contact with others — regardless of whether they've tested positive or not, he said.
Federal strategic reserve no longer maintained
Ottawa ordered more than 811 million rapid tests throughout the pandemic, at a cost of about $5 billion. Of those, roughly 680 million went to provincial and territorial rapid testing programs.The free COVID-19 rapid tests commonly come in boxes of five. (Jeff McIntosh/The Canadian Press)
A federal strategic reserve of rapid tests was maintained until Dec. 31, to ensure tests were readily available in Canada in case of future COVID-19 waves or an increase in demand, said Janveau.
While that reserve is no longer maintained, "provinces and territories have continued to receive rapid tests from the federal inventory upon request and while supplies last," he said.
Janveau did not say how many tests have expired to date, or at what cost.
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #713
AstraZeneca withdrawing Covid vaccine, months after admitting rare side effect
Company says decision is purely commercial as jab has been superseded by alternatives
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #714

Addiction Medication Offers New Hope for Long COVID Patients - Neuroscience News
Researchers identified a potential treatment for long COVID by restoring the function of ion channels in immune cells using low-dose Naltrexone.
Addiction Medication Offers New Hope for Long COVID Patients
May 7, 2024
Summary: Researchers identified a potential treatment for long COVID by restoring the function of ion channels in immune cells using low-dose Naltrexone. This discovery, detailed in Frontiers in Immunology, mirrors earlier findings with chronic fatigue syndrome (ME/CFS) patients, suggesting a common pathophysiological thread between the two conditions.The breakthrough could alleviate symptoms such as brain fog and muscle fatigue. Clinical trials for both long COVID and ME/CFS are set to begin, testing the efficacy of this repurposed drug.
Key Facts:
- Ion Channel Restoration: The study focuses on restoring ion channel function in immune cells, crucial for regulating bodily processes and alleviating symptoms.
- Repurposing Naltrexone: Naltrexone, a drug commonly used for opioid addiction, has shown promise in preliminary studies and anecdotal reports for improving ion channel function.
- Upcoming Clinical Trials: Griffith University is launching two clinical trials to assess the effectiveness of low-dose Naltrexone in treating both long COVID and ME/CFS patients.
Source: Griffith University
Researchers from Griffith University’s National Center for Neuroimmunology and Emerging Diseases (NCNED) have made a discovery that could bring relief to those struggling with long COVID.
In a world-first finding, they’ve identified a way to restore the faulty function of ion channels on immune cells using a well-known drug typically used for other medical purposes.
The breakthrough, published in the journal Frontiers in Immunology, builds on previous research showing long COVID patients share similar issues with ion channels as those with chronic fatigue syndrome (also known as myalgic encephalomyelitis or ME/CFS).

This drug, typically used for opioid addiction, has shown promising results in restoring ion channel function in previous research and in anecdotal reports from patients. Credit: Neuroscience News
The team had previously shown success in restoring ion channel function in ME/CFS patients using a drug called Naltrexone, and now they’ve achieved similar results with long COVID patients.
First author Ph.D. candidate Etianne Sasso said the research team had previously reported restoring the function of these ion channels of immune cells in laboratory trials.
“Ion channels are integral membrane proteins that facilitate the passage of ions (charged particles) across the cell membrane,” Sasso said.
“We found that by restoring the function of these ion channels, important ions such as calcium were again able to move in and out of immune cells, controlling many of the body’s biological processes.”
This breakthrough offers hope for alleviating various ME/CFS symptoms, including brain fog, muscle fatigue, and issues with the cardiovascular and gastrointestinal systems.
Professor Sonya Marshall-Gradisnik, senior author and Director of NCNED, said the significance of this discovery, achieved through the gold standard test called electrophysiology, will help in better understanding long COVID and ME/CFS paving the way for potential therapies.
The NCNED is preparing to launch two clinical trials, one for long COVID and another for ME/CFS, testing the effectiveness of low-dose Naltrexone.
This drug, typically used for opioid addiction, has shown promising results in restoring ion channel function in previous research and in anecdotal reports from patients.
“We will be undertaking two clinical trials testing the efficacy of low dose naltrexone where the first will be in long COVID patients while the second trial will, for the first time, be in ME/CFS patients,” Professor Marshall-Gradisnik said.
“Should these trials prove successful, it could mean a vastly improved quality of life for countless individuals struggling with long COVID and ME/CFS.”
About this neuropharmacology and long-COVID research news
Author: Etianne Sasso
Source: Griffith University
Contact: Etianne Sasso – Griffith University
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Investigation into the restoration of TRPM3 ion channel activity in post-COVID-19 condition: a potential pharmacotherapeutic target” by Etianne Sasso et al. Frontiers in Immunology
Abstract
Investigation into the restoration of TRPM3 ion channel activity in post-COVID-19 condition: a potential pharmacotherapeutic target
Introduction:
Recently, we reported that post COVID-19 condition patients also have Transient Receptor Potential Melastatin 3 (TRPM3) ion channel dysfunction, a potential biomarker reported in natural killer (NK) cells from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) patients. As there is no universal treatment for post COVID-19 condition, knowledge of ME/CFS may provide advances to investigate therapeutic targets.
Naltrexone hydrochloride (NTX) has been demonstrated to be beneficial as a pharmacological intervention for ME/CFS patients and experimental investigations have shown NTX restored TRPM3 function in NK cells. This research aimed to: i) validate impaired TRPM3 ion channel function in post COVID-19 condition patients compared with ME/CFS; and ii) investigate NTX effects on TRPM3 ion channel activity in post COVID-19 condition patients.
Methods:
Whole-cell patch-clamp was performed to characterize TRPM3 ion channel activity in freshly isolated NK cells of post COVID-19 condition (N = 9; 40.56 ± 11.26 years), ME/CFS (N = 9; 39.33 ± 9.80 years) and healthy controls (HC) (N = 9; 45.22 ± 9.67 years). NTX effects were assessed on post COVID-19 condition (N = 9; 40.56 ± 11.26 years) and HC (N = 7; 45.43 ± 10.50 years) where NK cells were incubated for 24 hours in two protocols: treated with 200 µM NTX, or non-treated; TRPM3 channel function was assessed with patch-clamp protocol.
Results:
This investigation confirmed impaired TRPM3 ion channel function in NK cells from post COVID-19 condition and ME/CFS patients. Importantly, PregS-induced TRPM3 currents were significantly restored in NTX-treated NK cells from post COVID-19 condition compared with HC. Furthermore, the sensitivity of NK cells to ononetin was not significantly different between post COVID-19 condition and HC after treatment with NTX.
Discussion:
Our findings provide further evidence identifying similarities of TRPM3 ion channel dysfunction between ME/CFS and post COVID-19 condition patients. This study also reports, for the first time, TRPM3 ion channel activity was restored in NK cells isolated from post COVID-19 condition patients after in vitro treatment with NTX.
The TRPM3 restoration consequently may re-establish TRPM3-dependent calcium (Ca2+) influx. This investigation proposes NTX as a potential therapeutic intervention and TRPM3 as a treatment biomarker for post COVID-19 condition.
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #715
@Sharma Ji
Oops. No more AstraZeneca.



 A vial labelled "AstraZeneca COVID-19 Vaccine" is seen in this illustration taken January 16, 2022.
A vial labelled "AstraZeneca COVID-19 Vaccine" is seen in this illustration taken January 16, 2022.
STORY CONTINUES BELOW THESE SALTWIRE VIDEOS
Bud the Spud hits the road | SaltWire

Watch on
(Reuters) - Anglo-Swedish drugmaker AstraZeneca is withdrawing its Covid vaccine worldwide, The Telegraph reported on Tuesday.
The application to withdraw the vaccine was made on March 5 and came into effect on Tuesday, the report added.
(Reporting by Urvi Dugar in Bengaluru; Editing by Pooja Desai)
Oops. No more AstraZeneca.
AstraZeneca to withdraw Covid vaccine worldwide, The Telegraph reports
Reuters | Posted: 34 minutes ago | Updated: 4 minutes ago | 1 Min Read



STORY CONTINUES BELOW THESE SALTWIRE VIDEOS
Bud the Spud hits the road | SaltWire

Watch on
(Reuters) - Anglo-Swedish drugmaker AstraZeneca is withdrawing its Covid vaccine worldwide, The Telegraph reported on Tuesday.
The application to withdraw the vaccine was made on March 5 and came into effect on Tuesday, the report added.
(Reporting by Urvi Dugar in Bengaluru; Editing by Pooja Desai)
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #716

Study identifies signifiers of severe COVID-19 disease and death
Why do some people with COVID-19 experience little more than a sniffle while others end up on a ventilator? And among critically ill patients, why do some eventually recover while others do not?
MAY 7, 2024
Editors' notes
Study identifies signifiers of severe COVID-19 disease and death
by Isabella Backman, Yale University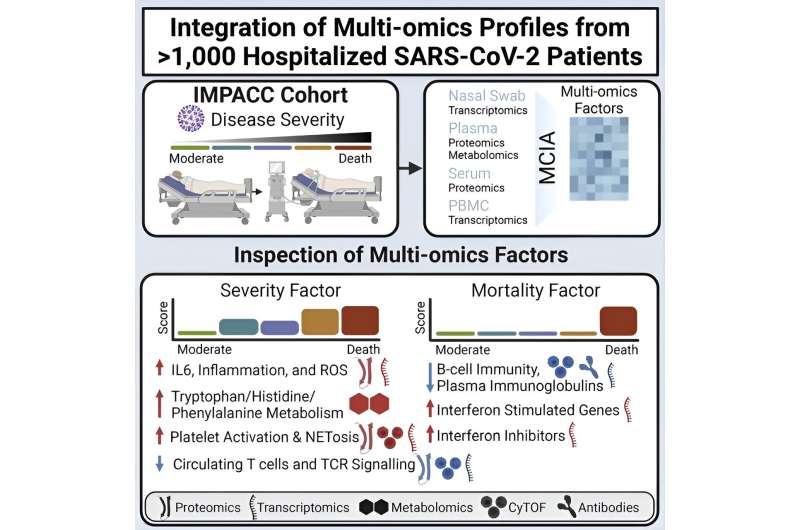
Why do some people with COVID-19 experience little more than a sniffle while others end up on a ventilator? And among critically ill patients, why do some eventually recover while others do not?
A new study has unveiled clues for helping scientists predict who is most at risk for severe COVID-19, and among those who experience severe disease, who is most likely to survive. The researchers published their findings in the Journal of Clinical Investigation on May 1.
The study drew on data from the Immunophenotyping Assessment in a COVID-19 Cohort (IMPACC) study, a partnership between the National Institute of Allergy and Infectious Diseases (NIAID) and 15 research institutions across the country, including Yale School of Medicine (YSM).
Collaborators conducted extensive analyses across many different aspects of the immune responses of more than 1,000 patients across the country. They assessed samples during hospitalization and for up to a year post-hospitalization to better understand the disease's heterogeneity.
The IMPACC multiomics approach, which combines multiple "omics" such as genomics, proteomics, and transcriptomics, is one of the largest and most comprehensive analyses to date.
COVID-19 has a vast array of manifestations in patients. To identify distinguishing features leading to different patient responses, IMPACC is using a systems immunology approach pioneered by the NIAID Human Immunology Project Consortium (HIPC) of the National Institute of Allergy and Infectious Diseases (NIAID).
The HIPC is directed at Yale by IMPACC members Ruth R. Montgomery, Ph.D., professor of medicine and of epidemiology (microbial diseases) and associate dean for scientific affairs at YSM, and David A. Hafler, MD, chair and William S. and Lois Stiles Edgerly Professor of Neurology.
Steven Kleinstein, Ph.D., Anthony N. Brady Professor of Pathology at YSM, also a HIPC investigator, led the multi-site IMPACC data analysis working group to process the individual data types for integrated analysis by the consortium informatics experts.
"My group was tasked with taking the millions of datapoints from these 1,000-plus individuals and using modeling to elucidate reasons why different people respond to COVID-19 differently and the molecular mechanisms behind it," says Jeremy Gygi, a Ph.D. candidate in Yale's computational biology and bioinformatics program and first author of the study.
New models look for patterns linked to COVID-19 outcomes
In their latest study, Gygi and the team wanted to identify signatures associated with severe COVID-19 infection and mortality. Furthermore, they examined interactions of these hallmarks to better understand the underlying immune cascade that occurs in critical cases."We didn't just look at someone's genes, proteins, and metabolites separately," says Gygi. "Instead, we examined how transcriptomic, proteomic, and metabolomic profiles for an individual work together in order to explain an outcome."
"This could be the largest-scale COVID-19 study by far that has looked at so many different 'omics' simultaneously and over time," adds Leying Guan, Ph.D., assistant professor of biostatistics at the Yale School of Public Health and the study's senior author.
"These are unique aspects of our study and have enabled us to do more than what's been done in the previous literature on COVID-19 biomarkers."
To achieve this, the team leveraged the IMPACC dataset and a computational method known as latent factor modeling. These models helped the researchers identify coordinated patterns among the multitude of assays they studied.
Their models had two main tasks. First, they wanted to identify drivers of severe disease. They looked for predictors that associated with the cohort's five clinical trajectory groups, with five being the most severe, and with the distinct trajectories of disease. Second, among the most severe groups, the researchers also looked for signatures predictive of mortality.
"We were trying to separate those who needed hospitalization and ventilation and survived, and those who did not," says Gygi.
Study identifies signifiers of severe COVID-19 disease and death
The severity model identified multiple factors significantly associated with COVID-19 disease trajectory, including inflammation, T cell lymphopenia, and catabolism of the amino acid tryptophan.While many of these signatures had been identified in the COVID-19 literature, the new models added a temporal element to reveal how these hallmarks evolved over time and interacted with one another.
Among the two most severe groups, an elevated discoordination of interferon signaling, which plays a vital role in immune response, significantly predicted mortality.
"For the severity cohort, although the hallmarks we found were already well-known, we identified an additional layer of interaction," says Guan. "Among the mortality cohort, we found an important type of dysregulation [of interferon signaling] that may characterize the fate of hospitalized patients."
This study is a significant achievement and an exciting starting point, the researchers say, and they have plans to build on this work to better understand more aspects of COVID-19. For example, they hope to use similar modeling techniques to gain a better understanding of long COVID and how it develops after an acute infection.
By learning more about the intricacies of COVID-19's underlying mechanisms, they hope to pave the way for new insights into more effective treatments for both acute and lingering disease.
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #717

What to know about the FliRT COVID variants
Here's what to know about the FliRT COVID variants
WHAT TO KNOW ABOUT THE FLIRT COVID VARIANTS
Here's what to know about the FliRT COVID variants

Written by Kaleb A. Brown, News Staff Writer who ties events you care about to products you need.
Updated May 7, 2024
Recommendations are independently chosen by Reviewed's editors. Purchases made through the links below may earn us and our publishing partners a commission.
It’s been over four years since the global outbreak of COVID-19 and while many are under the assumption that the pandemic is over, it’s important to remain vigilant. Case-in-point: a new family of COVID-19 variants — FliRT COVID — are currently making the rounds. These two variants, KP.1.1 and KP.2, together make up around 33% of new COVID-19 cases. These variants may have the capacity to spread more easily than older variants. While the news is troubling, there is no need to panic. Here is everything we know so far these new COVID-19 variants and ways you can stay safe.
What are the new FLiRT COVID variants?
FliRT is a family of omicron variant JN.1 offshoots consisting of the KP.1.1 and KP.2 variants. According to the Centers for Disease Control and Prevention (CDC), KP.2 is responsible for around 25% of new COVID-19 infections while KP.1.1 is responsible for about 7.5%.There is research that suggests that the FliRT variants spread more readily due to a mutation in the spike proteins responsible for transmission. This is a common occurrence for COVID-19 variants, which mutate to better evade immunity.
What are symptoms of the new FLiRT COVID variants?
As of this writing, symptoms of the FliRT COVID variants do not appear to differ from those of other variants. These symptoms include:- Fever or chills
- Cough
- Sore throat
- Congestion or runny nose
- Headache
- Muscle aches
- Difficulty breathing
- Fatigue
- New loss of taste or smell
- "Brain fog" (feeling less wakeful and aware)
- Gastrointestinal symptoms (upset stomach, mild diarrhea, vomiting)
What you need to protect yourself from COVID FliRT
Face mask
KN95 Face Mask, Reusable & Disposable Masks, 10 Pack
KN95 masks such as these filter 95% of particles, including the ones responsible for transmitting COVID-19.
Buy Now
Wearing a mask remains one of the most effective ways to protect yourself from contracting COVID-19. The best masks you can buy are N95 and KN95 masks, both of which filter 95% of the particles responsible for transmitting COVID-19. When shopping for masks online, do your due diligence to avoid the myriad of fakes.
Air purifier
BLUEAIR Air Purifier
Air purifiers can remove harmful air particles from your home, including ones that spread COVID-19.
Buy Now
Make sure the air in your home is free from COVID-19 by running a quality, HEPA air purifier. The best air purifier we’ve tested is the Blueair Blue Pure 311i Max. Beyond its quality HEPA filter, we love this air purifier because it’s quiet.
Hand sanitizer
Purell Advanced Hand Sanitizer Refreshing Gel
Disinfect your hands with hand sanitizer that contains at least 60% alcohol.
Buy Now
Another way to curb the spread of the FLiRT COVID-19 variants is to stay on top of your hand hygiene. This is because you can contract COVID by touching your hand to your eyes, nose, or mouth after touching a surface coated with COVID-19 particles. While the best way to disinfect your hands is to use soap and warm water, using hand sanitizer with at least 60% alcohol will work in a pinch.
Disinfectant wipes
Clorox Disinfecting Wipes Value Pack
Another way to kill germs is to use plenty of disinfectant wipes.
Buy Now
Disinfect surfaces to kill any viral particulates that may be lurking on them. Wipes will be most helpful on surfaces that are touched open such as doorknobs and light switches.
Thermometer
Vicks SpeedRead
Running a fever is one of the many symptoms of COVID-19.
Buy Now
If you start feeling under the weather, one way to see if you’ve contacted COVID-19 is by using a thermometer, as one of the symptoms is a running fever. One of the best thermometers to buy is Vicks SpeedRead digital thermometer, which is inexpensive, safe to wash in water, and has color-coded readings.
COVID-19 at home test
BinaxNOW COVID-19 Antigen Self Test
An at-home test can quickly tell you whether or not you have COVID-19.
Buy Now
Where are the FLiRT COVID variants spreading?
As of this writing, the FliRT variants are spreading evenly across the country — there isn’t a region where the variants are spreading more than others.What else to know about COVID-19 safety
Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #719
Duration and severity of COVID-19 symptoms among primary healthcare workers: A cross-sectional survey
Rongji Ma, Beier Lu, Yongjie Zhang, Ya Shen, Jinshui Xu, Hualing Chen, Yongkang Qian, Pengcheng Miao, Biyun Xu, Haijian Guo, Bingwei Chen
First published: 07 May 2024
https://doi.org/10.1111/jan.16212
Rongji Ma and Beier Lu contributed equally to this study.
Read the full text
TOOLS
SHARE
Abstract
Aims
This study aims to investigate the epidemiological characteristics of COVID-19 infection among healthcare workers, including the severity, duration of infection, post-infection symptoms and related influencing factors.Methods
A self-administered questionnaire was utilized to assess the post-infection status of primary healthcare workers in Jiangsu Province. The questionnaire collected information on demographic characteristics, lifestyle habits, post-infection clinical manifestations, work environment and recovery time of the respondents. Customized outcome events were selected as dependent variables and logistic regression models were employed to analyse the risk factors. Phi-coefficient was used to describe the relationship between post-infection symptoms.Results
The analysis revealed that several factors, such as female, older age, obesity, previous medical history, exposure to high-risk environments and stress, were associated with a higher likelihood of experiencing more severe outcomes. On the other hand, vaccination and regular exercise were found to contribute to an earlier resolution of the infection. Among the post-infection symptoms, cough, malaise and muscle aches were the most frequently reported. Overall, there was a weak association among symptoms persisting beyond 14 days, with only cough and malaise, malaise and dizziness and headache showing a stronger correlation.Conclusion
The study findings indicate that the overall severity of the first wave of infection, following the complete lifting of restrictions in China, was low. The impact on primary healthcare workers was limited, and the post-infection symptoms exhibited similarity to those observed in other countries. It is important to highlight that these conclusions are specifically relevant to the population infected with the Omicron variant.Impacts
This study helps to grasp the impacts of the first wave of COVID-19 infections on healthcare workers in China after the national lockdown was lifted.Patients
Primary healthcare workers in Jiangsu Province, including doctors, nurses, pharmacists and other personnel from primary healthcare units such as community health service centres and health centres.CONFLICT OF INTEREST STATEMENT
The authors declared they had no conflicts of interest.Yommie
SpeedLimited
- Oct 2, 2013
- 64,203
- 37,190
- Country of Origin

- Country of Residence

- Thread starter
- #720
COVID-19 kills 2 more in N.B., child and youth hospitalized
COVID activity remains moderate, Respiratory Watch report says, as NACI issues fall vaccine guidance

Bobbi-Jean MacKinnon · CBC News · Posted: May 07, 2024 1:15 PM EDT | Last Updated: May 7

An updated COVID-19 vaccine to replace the current XBB.1.5 vaccine may be available this fall, 'depending on the epidemiology of SARS-CoV-2 and recommendations of international advisory groups expected in mid-spring 2024,' the National Advisory Committee on Immunization said. (Ryan Remiorz/The Canadian Press)
Social Sharing
- X
comments
COVID-19 has killed two more New Brunswickers, while a child under four and a youth aged five to 19 are among the 19 people hospitalized for or with the virus, data released by the province Tuesday shows.
COVID activity remains moderate, and most indicators remained stable during the reporting period, April 21 to April 27, the Respiratory Watch report says.
The report comes just days after the National Advisory Committee on Immunization, known as NACI, issued its latest guidance on fall COVID-19 vaccines.
A fall dose is "strongly recommended" for people at higher risk of infection or severe disease, including seniors, people with underlying medical conditions, and those who provide essential community services, NACI said.
People aged six months or older who are not at increased risk may receive a fall dose, NACI said.
CBC News asked the Department of Health for comment about its fall vaccine plans, but did not receive a response.

NACI said there isn't sufficient data yet to determine the best time to start fall COVID-19 vaccinations, but preliminary observations from previous seasons suggest COVID activity began to increase before fall campaigns were launched. Last year, the national percent positivity began to increase in mid-August, it said. (Jonathan Hayward/The Canadian Press)
NACI "emphasizes the benefits of available vaccines for COVID-19 protection, and particularly for those most at risk of severe illness, as we know that protection against severe illness due to COVID-19 can wane over time," chair Dr. Robyn Harrison said in a statement Friday.
"An updated COVID-19 vaccine formulation may also be available by the fall that would better target the currently circulating strains," she said.
Spring boosters have been available since April 2 to New Brunswickers considered most at risk of severe illness.
Nearly 2,000 COVID vaccines were administered in the past week, raising the total to 155,620 since Oct. 4, according to figures from the department.
The spring doses will be available until June 15, the department has said.
4 outbreaks, 29 new cases
The two people who died from COVID during the reporting week were aged 45 to 64 and 65 or older.Their deaths raise the provincial pandemic total to at least 1,034. The actual number is unclear because the Department of Health counts only people who die in hospital as COVID deaths.
The 19 people who were hospitalized either because of COVID or for something else and later tested positive for the virus is up from 17 in the previous report. One person was admitted to intensive care, the same number as a week ago.
In addition to the child and youth, those admitted to hospital included one person aged 20 to 44, two people aged 45 to 64 and 14 aged 65 or older, one of whom required intensive care.

PCR lab tests are restricted in New Brunswick to those with symptoms who have a referral from a primary care provider and for whom the outcome will 'directly influence treatment or care.' (Kamran Jebreili/The Associated Press)
The number of lab-confirmed COVID outbreaks doubled to four since the last report. One was in a nursing home and the other three were in facilities listed only as "other."
Twenty-nine new cases of COVID were confirmed through polymerase chain reaction (PCR) lab tests, down from 31.
The positivity rate — or the percentage of lab tests performed that produced a positive result — is four per cent, unchanged.
Flu sends child and youth to hospital
No influenza deaths were reported between April 21 and April 27, but the flu sent seven people to hospital, up from six the previous week.Among those admitted to hospital were a child under four, a youth aged five to 19, one person aged 20 to 44, one aged 45 to 64 and three aged 65 or older.
Influenza activity remains relatively stable, the report says.
Forty-two new flu cases were confirmed by lab tests, down from 59, with a positivity rate of five per cent, down from seven.
Eleven of the new cases were influenza A and 31 were influenza B.
Since the respiratory season began on Aug. 27, there have been 3,630 confirmed flu cases confirmed.
A total of 224,109 New Brunswickers have been vaccinated against the flu, as of Tuesday, an increase of 146 from a week ago, figures from the department show.
Horizon and Vitalité
Horizon Health Network has eight active COVID admissions, as of Saturday, down from 10 last week, according to its latest COVID report.None are in intensive care, down from one.
Two health-care workers are off the job, after they tested positive for COVID-19, compared to one a week ago, the report says.
Horizon's COVID-19 outbreak page shows one outbreak, as of Tuesday, at the Moncton Hospital's cardiology unit.
Vitalité Health Network updates its COVID report only monthly, with the next update expected on May 28.
It updates its outbreaks page more frequently, however, and lists one outbreak, as of Tuesday, at the Chaleur Regional Hospital in Bathurst, on the general medical unit.
Users who are viewing this thread
Total: 1 (members: 0, guests: 1)
Pakistan Defence Latest
-
Breaking news: Malakand 10 diplomates from different countries attacked...All safe (4 Viewers)
- Latest: Maarkhoor
-
-
Durga Puja gift: Bangladesh interim government to export 3,000 tonnes of hilsa to Bengal (7 Viewers)
- Latest: MNZGamerX
-
Iran has requested the Pakistan Air Force to provide training for its fighter pilots! (10 Viewers)
- Latest: MastanKhan
-
Country Watch Latest
Latest Posts
-
-
Breaking news: Malakand 10 diplomates from different countries attacked...All safe (4 Viewers)
- Latest: Maarkhoor
-
-
'Indus Water Treaty Not feasible to maintain': India serves notice to Pakistan, seeks Modification. (7 Viewers)
- Latest: vasanthm
-
Durga Puja gift: Bangladesh interim government to export 3,000 tonnes of hilsa to Bengal (7 Viewers)
- Latest: MNZGamerX

