Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Covid-19 News and Discussions
- Thread starter Yommie
- Start date
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,223

Moderna Plunges as Sales View Disappoints, Research Budget Cut
Moderna Inc. aims to reduce its research and development budget by about 20% over the next three years as the biotech tries to find a path to profitability following disappointing vaccine sales.
Moderna Plunges as Sales View Disappoints, Research Budget Cut
By Gerry SmithSeptember 12, 2024 at 6:22AM EDT
Hot Picks
(Bloomberg) -- Moderna Inc. said it aims to reduce its research and development budget by about 20% over the next three years as it tries to find a path to profitability following disappointing vaccine sales.
The biotech is discontinuing five programs in its pipeline and slowing some late-stage studies of treatments for latent and rare diseases to achieve the $1.1 billion cut from its annual R&D budget by 2027, according to a statement Thursday.
The shares fell as much as 19% at the New York market open. They had lost 20% this year through Wednesday’s close.
The company’s revenue projection for next year was below what analysts expected and it announced it was pushing back its target to break even from 2026 to 2028. Questions remain about whether Moderna has enough cash to fund its plans to break even without raising additional equity, Jefferies analyst Michael Yee said in a note.
“Investors are unlikely to believe this until further credibility,” he said.
What Bloomberg Intelligence Says:
Uncertainty over Moderna’s revenue prospects explains the reduction in 2024-28 R&D spending to $20.8 billion (from $25 billion), with deprioritized assets not a major setback. As we expected, the FDA has discouraged filing mRNA-4157’s Phase II data in melanoma, with a later launch another risk to 2026-28 consensus sales.
— BI analyst Sam Fazeli. Reach the research here.
The Cambridge, Massachusetts-based company is slowing its pace of studying new treatments in part because of recent commercial challenges. The company sells two products — a vaccine for Covid and another for RSV. It projected sales of between $2.5 billion and $3.5 billion next year. It has said this year’s sales will range between $3 billion and $3.5 billion, down from its previous outlook of about $4 billion.
‘Financial Discipline’
“We are still dealing with a market of uncertainty,” Moderna Chief Financial Officer Jamey Mock said in an interview. “We hope that will settle out this year but we have to brace ourselves just in case vaccination rates continue to go down.”
Mock said the R&D cuts are a sign that the company “is exercising financial discipline.”
Moderna needed to reduce its R&D budget in part because its clinical trials have succeeded and later-stage studies require more funding, he said. Moderna expects 10 products to get approved over the next three years.
“We do recognize the need to pace ourselves because there is now this huge bolus of important medicines to get approved,” Moderna President Stephen Hoge said in an interview.
The company is known for spending heavily on R&D, often more than its peers as a percentage of sales. The spending has been fueled by the belief that its mRNA technology could effectively treat and prevent a range of illnesses, from flu to cancer. But the sharp decline of its Covid vaccine business is forcing the company to rein in some of its ambitions.
Cancer Program
One of its biggest hopes is its cancer program. Late last year, Moderna and its partner, Merck & Co., said its melanoma vaccine helped prevent the recurrence of severe skin cancer for three years.
Moderna executives have hoped to file for faster approval based on data from that mid-stage study. Moderna said initial feedback from US regulators “has not been supportive” of that, and the company and Merck are focused on its late-stage trial.
The company said it plans to increase its research and development investments in oncology.
It’s also no longer pursuing an accelerated approval for its standalone flu vaccine. Instead, it will focus on filing for approval this year for its vaccine that combines flu and Covid protection.
“We just think that has the chance to be a bigger product and have a larger impact,” Mock said.
(Adds analyst comments from fourth paragraph, updates shares.)
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,225
Covid Situation Report: Sep 12, 2024
Update on Covid providing information on prevalence and hospital admissions for England and its regions. This post is best viewed using the browser or Substack app.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,226
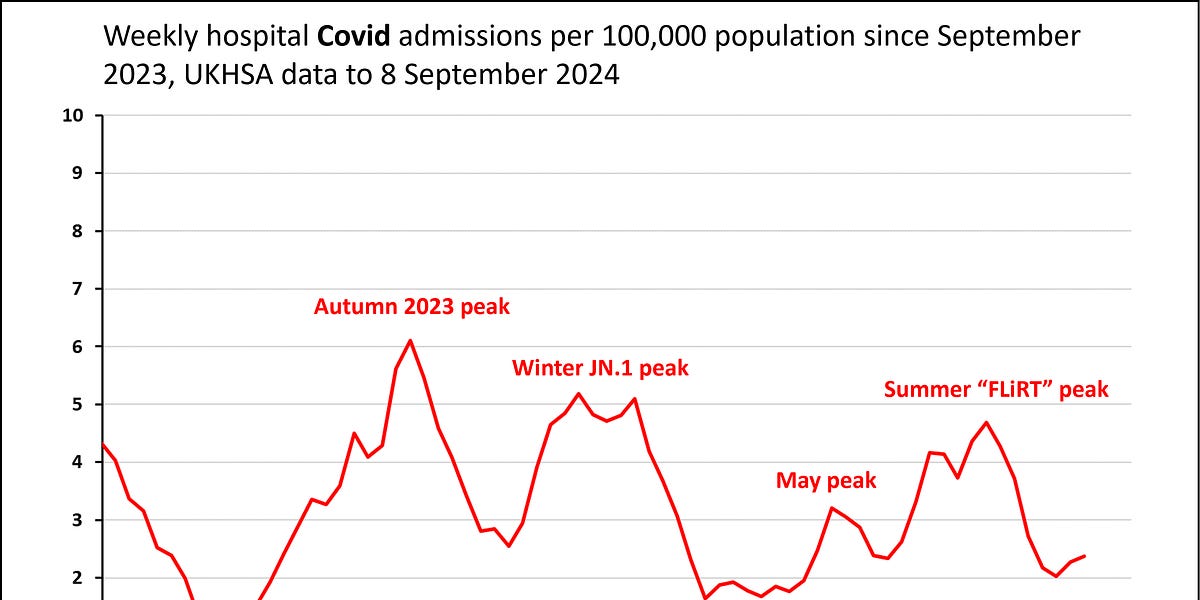
Quick update on Covid levels in England - looks like cases rising (slowly) this month
There is not much data around.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,227
U.S. to resume mandatory reporting of COVID hospitalizations
Hospitals in the United States will soon be required to report hospital admissions related to COVID-19, restoring a mandate which was lifted earlier this year, according to federal officials. The new rules are due to take effect on November 1. Hospital data about COVID-19 was often cited as an...
U.S. to resume mandatory reporting of COVID hospitalizations
Published on
September 6, 2024
By
BNO News

Hospitals in the United States will soon be required to report hospital admissions related to COVID-19, restoring a mandate which was lifted earlier this year, according to federal officials. The new rules are due to take effect on November 1.
Hospital data about COVID-19 was often cited as an alternative when many states stopped releasing COVID-related data, including case counts. But those reports became voluntary on May 1, with only 33% of hospitals choosing to participate.
Beginning November 1, hospitals will once again be required to report COVID-19 data, as well as information about influenza and RSV, according to the Centers for Medicare & Medicaid Services (CMS) at the U.S. Department of Health.
“The information required to report includes confirmed infections of respiratory illnesses, including COVID-19, influenza, and RSV, among hospitalized patients; hospital bed census and capacity; and limited patient demographic information, including age,” a CMS spokesperson told BNO News.
CMS is proposing updates on a weekly basis, though the final decision about the exact frequency is up to the Secretary of Health, Xavier Becerra. His office did not immediately respond when asked about the frequency of updates.
The U.S. is currently in the midst of a COVID summer wave with nearly 178,000 new cases reported last week alone, along with more than 1,000 deaths. Data from the 33% of hospitals participating in the voluntary survey showed 5,357 Americans in hospital with COVID-19. The actual number is believed to be higher.
So far this year, more than 4.9 million COVID cases have been reported across the U.S., causing at least 348,034 hospitalizations (limited data from May 1) and 38,563 deaths, according to BNO’s COVID data tracker.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,229
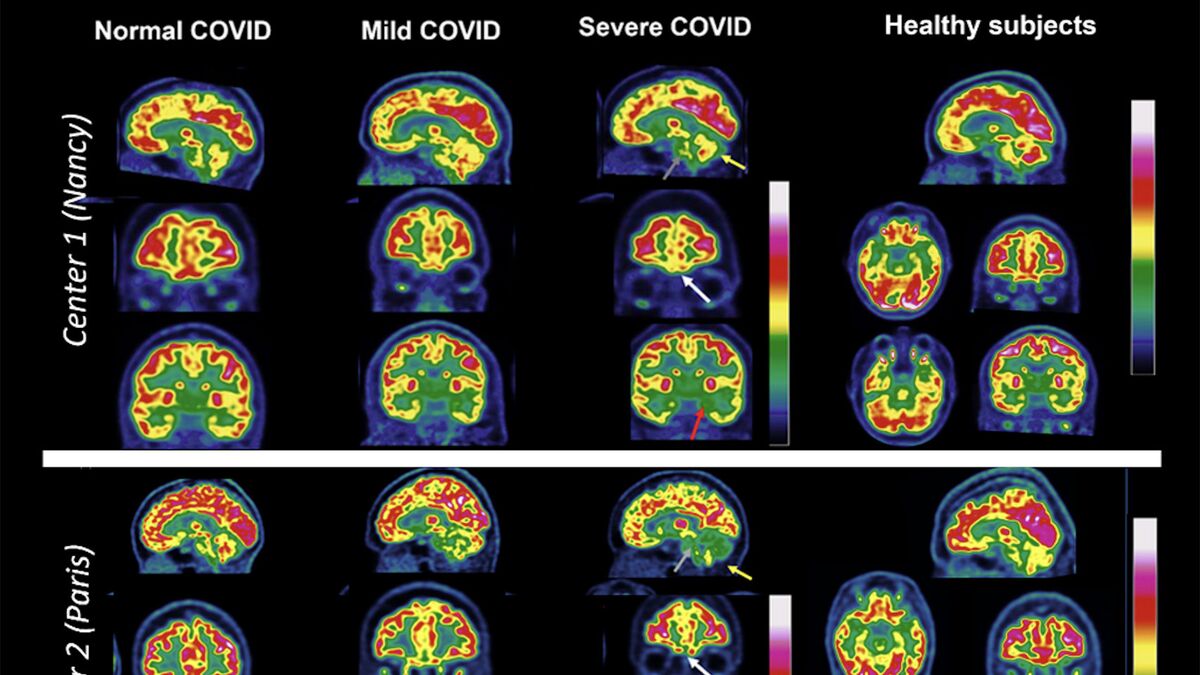
What We Know About Covid’s Impact on Your Brain
Scientists are worried that persisting cognitive issues may signal a coming surge of dementia and other mental conditions
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,230
Long-COVID Patients Show Abnormal Lung Perfusion Despite Normal CTs
A study found abnormal lung perfusion in long-COVID patients with normal CT scans, highlighting pulmonary vascular issues. At ERS 2024, researchers call for further investigation into the condition.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,233

Covid 19 update: 1041 new cases, 18 further deaths
The number of new cases of Covid-19 in New Zealand has decreased since last week, and the number of deaths attributed to the virus has dropped slightly.
Covid 19 update: 1041 new cases, 18 further deaths
11:10 am on 10 September 2024Share this
- Share on Twitter
- Share on Facebook
- Share via email
- Share on Reddit
- Share on Linked In

Photo: 123rf.com / Composite Image - RNZ
The number of new cases of Covid-19 in New Zealand has decreased since last week, and the number of deaths attributed to the virus has dropped slightly.
There were 1041 new cases of Covid-19 reported in New Zealand over the week to Sunday, and 18 further deaths attributed to the virus.
Of the new cases, 676 were reinfections.
There were 82 people in hospital, with none in intensive care.
The highest number of new cases were in Waitematā (152), followed by Canterbury with 99.
The previous week there were 1425 new cases with 19 deaths attributed to the virus.
There were 92 cases admitted to hospital.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,234
Austrian woman is found guilty of fatally infecting her neighbor with COVID-19
A woman in Austria has been found guilty of fatally infecting her neighbor with COVID-19 in 2021, her second pandemic-related conviction in a year, according to local media.
Austrian woman is found guilty of fatally infecting her neighbor with COVID-19
FILE - People rest after receiving the vaccination against the new coronavirus in the ‘Austria Center Vienna’ in Vienna, Austria, Friday, April 9, 2021. (AP Photo/Lisa Leutner, File)
Updated 9:24 AM EDT, September 13, 2024
VIENNA (AP) — A woman in Austria was found guilty of fatally infecting her neighbor with COVID-19 in 2021, her second pandemic-related conviction in a year, according to local media. A judge sentenced the 54-year-old on Thursday to four months’ suspended imprisonment and an 800-euro fine ($886.75) for grossly negligent homicide.
The victim, who was also a cancer patient, died of pneumonia that was caused by the coronavirus, according to Austrian news agency APA. A virological report showed that the virus DNA matched both the deceased and the 54-year-old woman, proving that the defendant “almost 100 percent” transmitted it, an expert told the court.
“I feel sorry for you personally -- I think that something like this has probably happened hundreds of times,” the judge said Thursday. “But you are unlucky that an expert has determined with almost absolute certainty that it was an infection that came from you.”
While the judge issued the sentence Thursday, APA reported that the verdict isn’t yet final. The names of the victim and defendant were not released in line with Austrian privacy rules.
The woman was convicted of a COVID-related offense last summer, APA reported. The agency said she was sentenced to three months’ suspended imprisonment for intentionally endangering people through communicable diseases. But she was acquitted on the grossly negligent homicide charge at that time.
This week, the judge heard statements from the deceased’s family, who said there had been contact in a stairwell between the neighbors on Dec. 21, 2001 — when the defendant would already have known she had COVID-19. But she denied the meeting, saying she was too sick to get out of bed that day. She also said she believed she had bronchitis, which she typically gets every year.
But the woman’s doctor told police that the defendant had tested positive with a rapid test and told him that she “certainly won’t let herself be locked up” after the result.
Yommie
Elite Member
- Oct 2, 2013
- 63,876
- 37,180
- Country of Origin

- Country of Residence

- Thread starter
- #2,235
Florida discourages use of mRNA Covid vaccines in older adults
In updated guidance to health care providers, Florida health officials advise that "non-mRNA Covid-19 vaccines and treatment" should be prioritized.
Florida discourages use of mRNA Covid vaccines in older adults
In updated guidance to health care providers, Florida health officials advise that "non-mRNA Covid-19 vaccines and treatment" should be prioritized.
A nurse prepares the Pfizer-BioNTech Covid vaccine in Montreal.Andrej Ivanov / AFP - Getty Images file
By Berkeley Lovelace Jr.
Even as the Covid wave in Florida continues, Gov. Ron DeSantis’ administration is once again advising against the mRNA vaccines: this time in the most vulnerable residents.
In updated guidance for health care providers released Thursday, the Florida Health Department and state Surgeon General Joseph Ladapo questioned the safety and effectiveness of the mRNA Covid vaccines from Pfizer and Moderna, including for older adults and people with underlying health problems. “Any provider concerned about the health risks associated with Covid-19 for patients over the age of 65 or with underlying health conditions should prioritize patient access to non-mRNA Covid-19 vaccines and treatment,” according to the state guidance.
Standing in opposition to advice from federal health agencies and other medical experts about the safety of the Covid vaccines, the Florida Health Department said the recommendation was based on high rates of immunity from prior infections and “currently available data.”
Without noting the high risk of serious Covid infection or hospitalization for older adults, the guidance listed safety concerns for the mRNA vaccines, including the risk of a rare heart condition called myocarditis, as well as the risk of POTS, or postural orthostatic tachycardia syndrome, a debilitating heart condition.
Numerous studies have shown that both Pfizer and Moderna’s vaccines are indeed associated with a small but increased risk of myocarditis. However, most cases occur in young men and most people make a full recovery. Studies have also shown that the risk of myocarditis is much higher with a Covid infection and is often more severe than the vaccine-associated condition.
A study published in Nature Cardiovascular Research also found that people diagnosed with Covid are five times more likely to develop POTS after infection than after Covid vaccination, emphasizing the importance of the vaccine.
Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia, said the Florida surgeon general's guidance is unnecessarily alarming people about the Covid vaccines.
"It's just such a dangerous game he plays," said Offit, who has served on the FDA's independent vaccine advisory committee. "You only have a roughly 1,000 times greater likelihood of dying [from Covid] if you're over 65 than if you're under 18."
"The mRNA vaccines are remarkably safe," he added.
New doses of the Pfizer and Moderna vaccines were approved by the Food and Drug Administration in August. They’re targeted to the KP.2 version of the steadily mutating virus and should provide good protection against severe illness, hospitalization and death, experts say.
The United States offers only one Covid vaccine that isn’t mRNA-based: Novavax. Novavax’s traditional protein-based shot offers an alternative vaccine technology to mRNA.
In a statement, a Novavax spokesperson said its updated vaccine is now available at thousands of retail and pharmacy locations nationwide.
The Florida Health Department did not immediately respond to a request for additional comment.
Dr. Isaac Bogoch, an infectious disease specialist at the University of Toronto, called Florida’s recommendation “unfortunate,” saying it could put older adults and people with underlying health conditions at risk.
“While the vaccine does not protect against infection nearly as well as it once did, there is very good data demonstrating how Covid vaccines can reduce the risk of severe manifestations of the virus in those who are at greatest risk,” said Boguch, who has no ties to the vaccine makers.
Covid cases remain high in the U.S. following a summer wave. Wastewater data collected between Aug. 25 and Aug. 31 shows that 23 states -- including Florida -- are reporting “very high” levels of the virus, according to the CDC. Emergency department visits and hospitalizations remain elevated as well, although they’re showing signs of declining. In Florida nursing homes, after declining from summer highs, Covid infections are ticking up again, according to CDC data.
The CDC currently recommends everyone ages 6 months and older get an updated Covid vaccine this fall from any of the three options.
This isn’t the first time Ladapo has ignored CDC guidance. Last year, Ladapo also recommended that Florida residents under the age of 65 not get the mRNA Covid vaccines, citing widespread immunity and “questions we have about safety and about effectiveness.”
“My judgment is that it’s not a good decision for young people and for people who are not at high risk at this point in the pandemic,” he said.
Users who are viewing this thread
Total: 1 (members: 0, guests: 1)
Pakistan Defence Latest
Country Watch Latest
-
Here Are Seven Steps That Union Government Must Take To Secure The ‘Chicken’s Neck’ Corridor (11 Viewers)
- Latest: Krptonite
-
-
-
-
Latest Posts
-
-
Hundreds of Hezbollah fighters injured after their pagers/phones explode in presumed Israeli operation (33 Viewers)
- Latest: nahtanbob
-
Here Are Seven Steps That Union Government Must Take To Secure The ‘Chicken’s Neck’ Corridor (11 Viewers)
- Latest: Krptonite
-
-
